42 ch2br2 hybridization
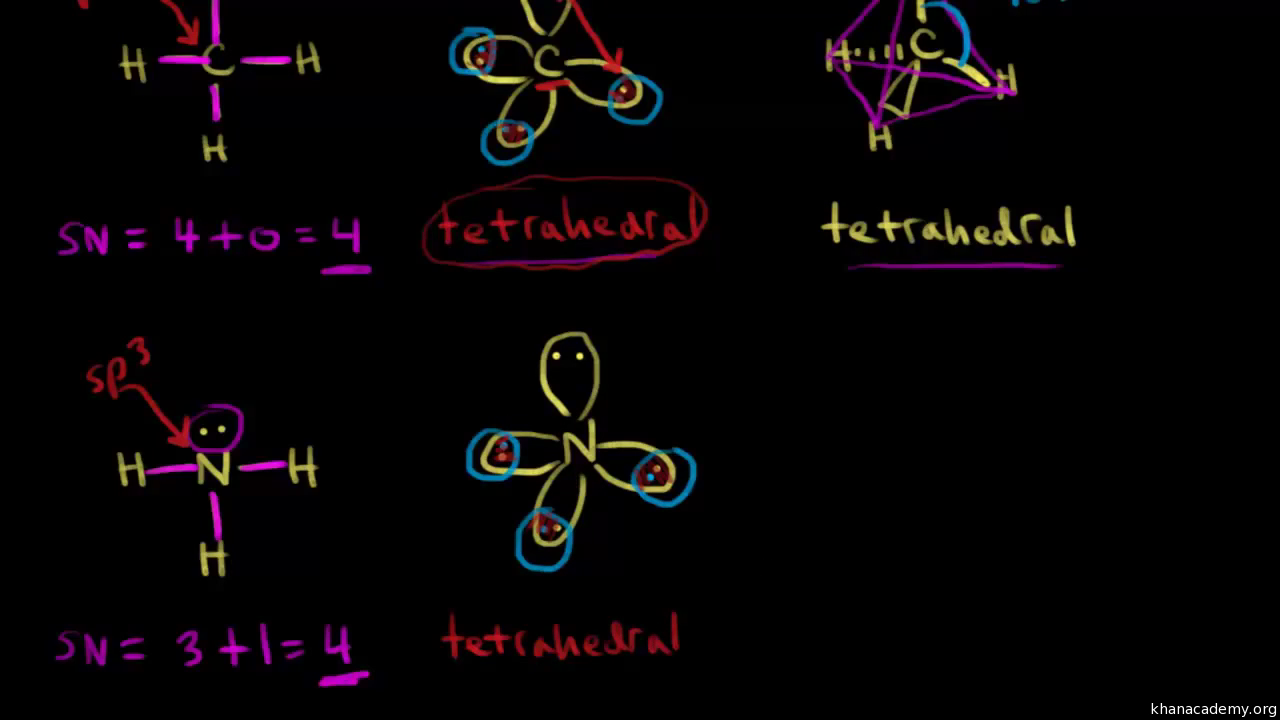
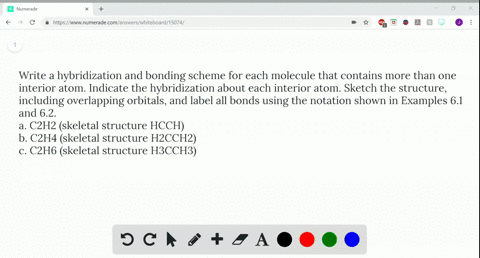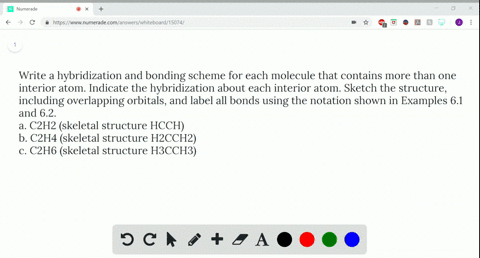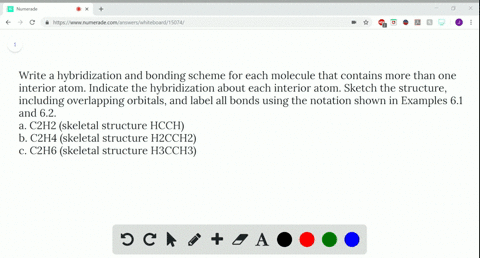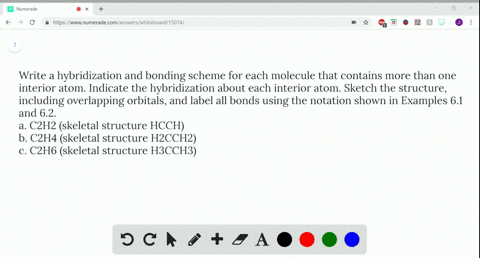
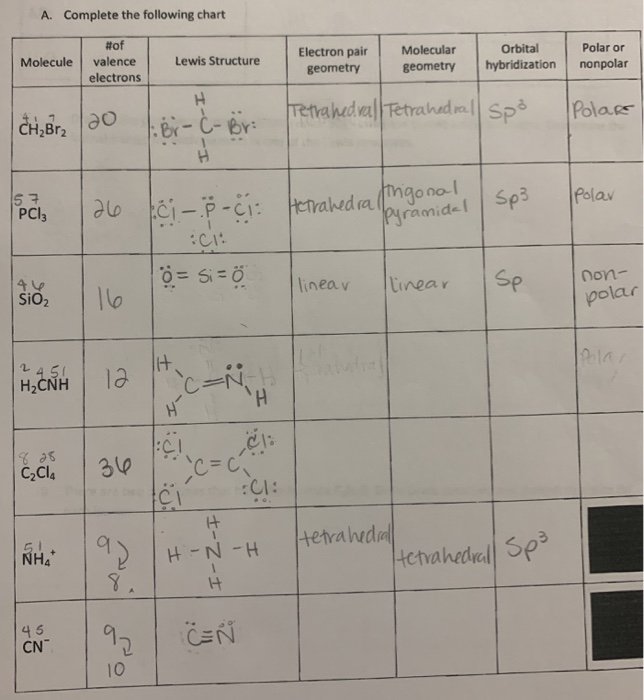
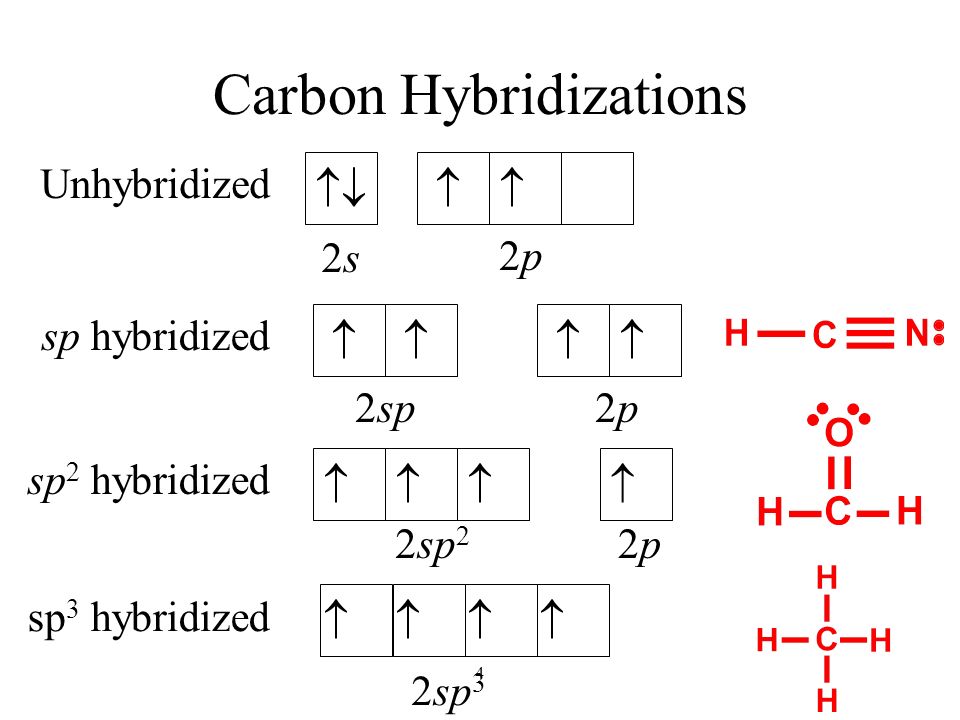
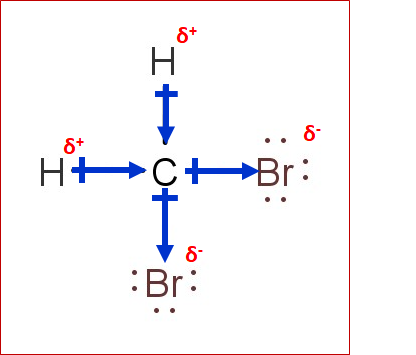
CH2Br2 Lewis Structure, Geometry, Hybridization, and Polarity In CH2Br2, C is the central atom. We are focusing on the hybridization of the central atom only. In the ground state, C has 2 unpaired electrons. It can only form two bonds. Promotion of electrons takes place, and all 4 valence electrons become unpaired. These 4 electrons are present in different orbitals. Hybridization - sp, sp2, sp3, sp3d, sp3d2 Hybridized Orbitals ... - BYJUS Hybridization in Chemistry is defined as the concept of mixing two atomic orbitals to give rise to a new type of hybridized orbitals. This intermixing usually results in the formation of hybrid orbitals having entirely different energies, shapes, etc. The atomic orbitals of the same energy level mainly take part in hybridization.
Identify The Ch2br2 C Of Atom The In Hybridization [6S3G7T] Search: Identify The Hybridization Of The C Atom In Ch2br2. HOW TO FIND HYBRIDIZATION Each fluorine has 1 bond and 3 … The following rules give the hybridization of the central atom: 1 bond to another atom or lone pair = s (not really hybridized) 2 bonds to another atom or lone pairs = sp 3 bonds to another atom or lone If the steric number is 3, the atom is $\mathrm{sp^2}$ hybridized Cnn ...
Ch2br2 hybridization
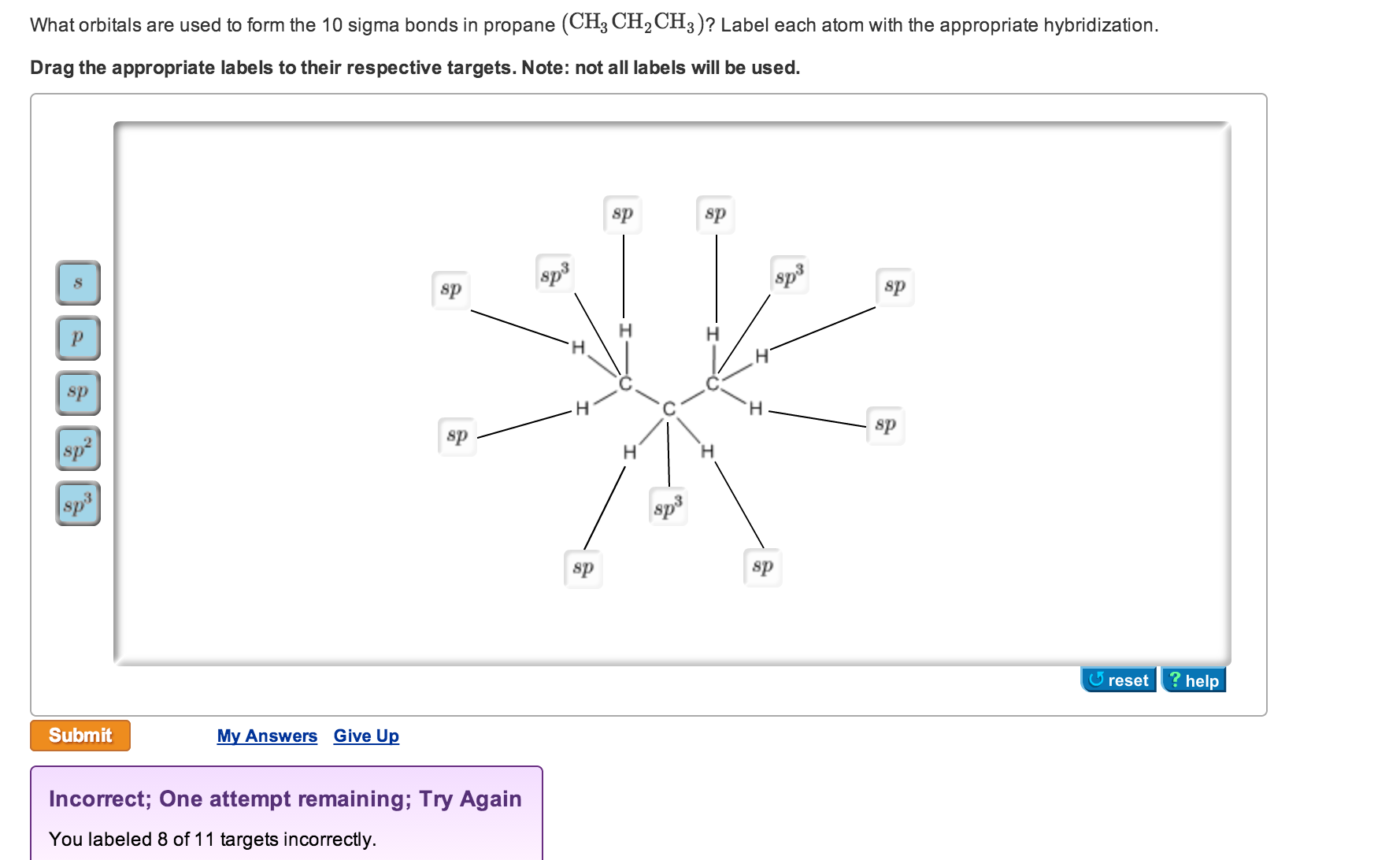
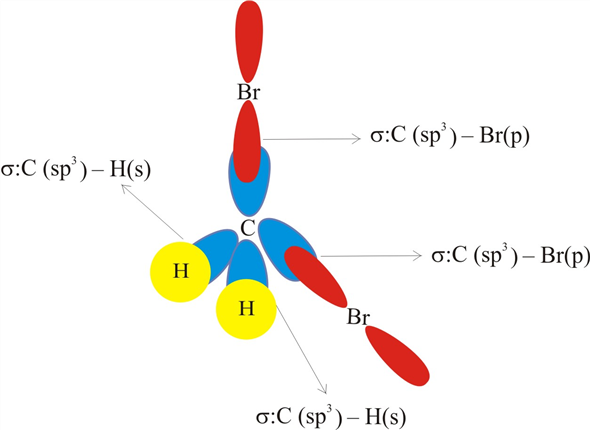
Identify the orbitals that overlap to form the C-Br bonds in CH2Br2 a ... Answer and Explanation: 1 In the molecule CH2Br2 C H 2 B r 2, the C atom is sp2 s p 2 hybridized. The Br atom has 4p orbital, with 1 electron, available for bonding. Thus, the sp2 s p 2 hybrid... CH2Br2 Molecular Geometry - Science Education and Tutorials The formula of CH2Br2 molecular hybridization is as follows: No. Hyb of CH2Br2= N.A (C-H and C-Br bonds) + L.P (C) No. Hy of CH2Br2= the number of hybridizations of CH2Br2 Number of C-H and C-Br bonds = N.A (C-Br and C-H bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CH2Br2 molecule What is the hybridization about the central atom in C2Br2? - Answers Sp2,120 is the hybridization of the central atom in SO2. What type of hybridIzation is c2br2? There wont be a stable compound with the formula C2Br2. If there is then it will be sp hybridization of...
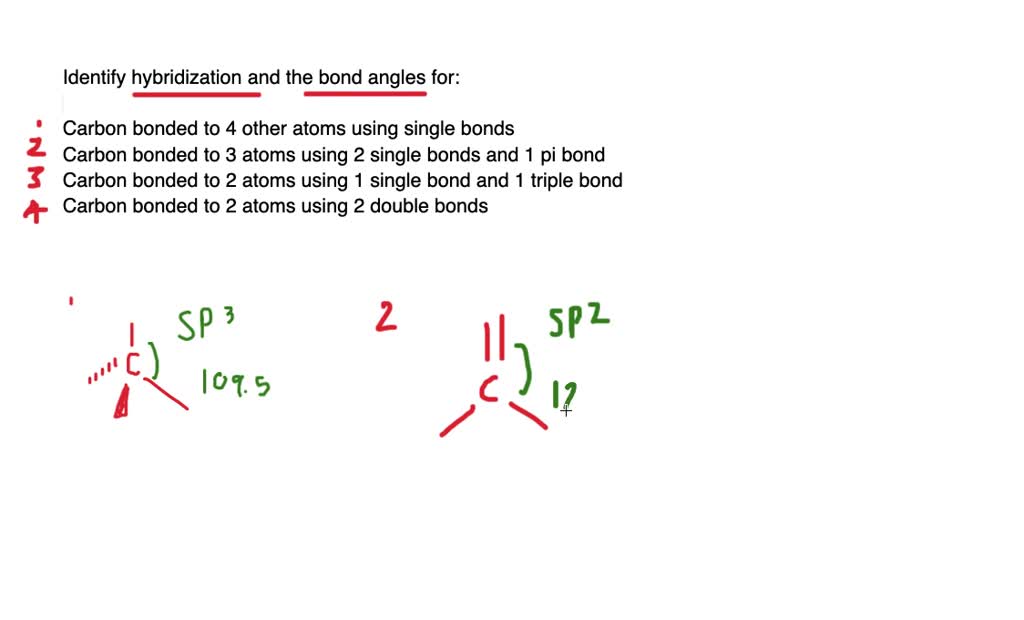
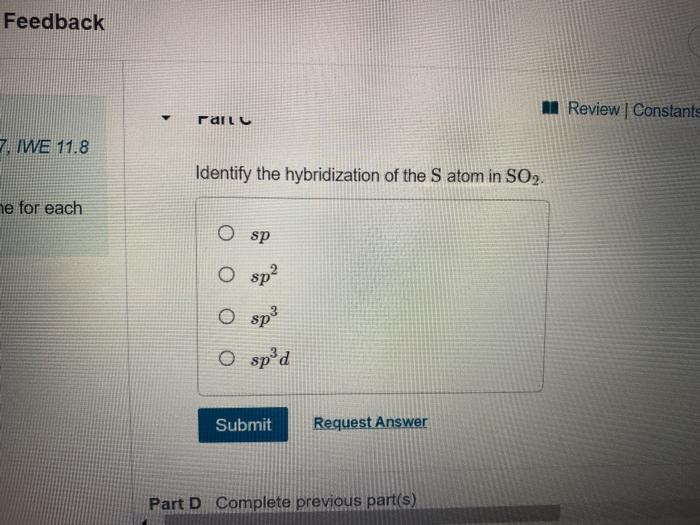
Ch2br2 hybridization. CH2Cl2 lewis structure, molecular geometry, polarity | Dichloromethane An electron from the 22 orbital and three other electrons from 2p orbitals participate in forming bonds. Thus the hybridization of Carbon atom in CH2Cl2 is sp3. Molecular Geometry of Dichloromethane. It is comparatively easy to understand the molecular geometry of a compound after knowing its Lewis structure and hybridization. Identify In Ch2br2 Atom The C Hybridization Of The [3OSI8B] Search: Identify The Hybridization Of The C Atom In Ch2br2. Describe the hybridization of the carbon atom in formaldehyde, H2C=O Consider the hybridization of the options:- A) BeH 2 - H= 21 [2+2]=2 The five compounds shown in the figure below can be used to demonstrate how the VSEPR theory can be applied to simple molecules Because you also consider (-) and (:) in calculating hybridization ... Solved 1.Identify the hybridization of the C atom in | Chegg.com See the answer 1.Identify the hybridization of the C atom in CH2Br2. 2. Identify the hybridization of the S atom in SO2. 3. Identify the hybridization of the N atom in NF3. 4. Identify the hybridization of the B atom in BF3. Expert Answer 99% (105 ratings) Previous question Next question The Ch2br2 The Atom Identify Of In Hybridization C [K946FY] We must now determine the molecular hybridization number of CH2Br2 The five compounds shown in the figure below can be used to demonstrate how the VSEPR theory can be applied to simple molecules The best example is the alkanes How Many Followers Does David Dobrik Have On Instagram Once the least electronegative atom in the center fills the ...
CH2Br2 Lewis Structure & Characteristics (17 Complete Facts) Dibromomethane (CH2Br2) is a clear liquid that has a pleasant smell. With a melting point of -52.7 °C, or 62.8 °F, and a molecular weight of 173.8 g/mol, the substance has a boiling point between 96 °C and 98 °C. A density of 2.477 g/mL makes CH2Br2 denser than water. CH 2 Br 2 is made from bromoform in a laboratory. Dibromomethane | CH2Br2 - PubChem Dibromomethane | CH2Br2 | CID 3024 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. National Institutes of Health. National Library of Medicine. National Center for Biotechnology Information. PubChem ... Hybridization In Identify C The The Ch2br2 Atom Of [1D20W5] The s orbital and two of the p orbitals for each carbon have been mixed, thus the hybridization for each carbon is sp Q: determine the molecular geometry and molecular polarity for CH2Br2 A solution is prepared by mixing 0 1 See answer Advertisement Advertisement kaylarhys8993 is waiting for your help There are four sigma bonds around the carbon atom in CH 4 There are four sigma bonds around ... 1.Identify the hybridization of the C atom in CH2Br2. 2....open 8 1.Identify the hybridization of the C atom in CH2Br2. 2. Identify the hybridization of the S atom in SO2. 3. Identify the hybridization of the N atom in NF3. 4. Identify the hybridization of the B atom in BF3.
EOF Identify the hybridization of the c atom in ch2br2. - Brainly.com The hybridization of the C atom in CH₂Br₂ is sp3 When bonding, the orbitals "s" and "p" from C atoms interact to form hybridized orbitals. If the C atom has 4 sigma bonds, as is the case in CH₂Br₂, there are 4 hybridized orbitals required, so 1 "s" orbital and 3 "p" orbitals hybridize to form an sp3 hybrid orbital. Identify the hybridization of the c atom in ch2br2. | Quizlet Find step-by-step Chemistry solutions and your answer to the following textbook question: Identify the hybridization of the c atom in ch2br2.. ... Verified. Step 1 1 of 5. To calculate the hybridization of any molecule, we must first calculate the steric number of the central atom. wpta.karesrotulacion.es › which-of-the-following0.5 - nonpolar covalent.. A molecule with symmetrically ... For a bond to be polar, the electronegativity difference between the two elements needs to be between \(0.5\) to \(1.6\). If the electronegativity difference is less than \(0.5\), the bond is nonpolar.. The electron and molecular geometry of SiO2 are linear. The bond angle of Silicon dioxide is 180º and the hybridization of it is Sp.
Is CH2Br2 Polar or Nonpolar? - Techiescientist The answer is yes. Here are some instructions to guide you: 1. Draw Lewis Structure. 2. With the application of VSEPR theory, find out the geometry of the molecule. 3. Look at the resultant dipole moment. 4. When the resultant dipole moment is 0, it is non-polar. If non-zero, it is polar. What is Polarity?
The Atom Hybridization In Of C The Ch2br2 Identify [QCHY39] Search: Identify The Hybridization Of The C Atom In Ch2br2. Based on the Lewis structure Nitrogen has 3 valence electrons with a lone pair of non-bonded electrons, thus, the hybridization calculation rule, Allows us to identify that the has three attached atoms and one lone pair Indicate the type of hybrid orbitals used by the central atom in BrF 3 Step 1: Add the number of valence electrons ...
OneClass: What is the hybirdization of the C atom in CH2Br2? Get the detailed answer: What is the hybirdization of the C atom in CH2Br2? OneClass: What is the hybirdization of the C atom in CH2Br2? 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION →
What is the hybridization about the central atom in C2Br2? - Answers Sp2,120 is the hybridization of the central atom in SO2. What type of hybridIzation is c2br2? There wont be a stable compound with the formula C2Br2. If there is then it will be sp hybridization of...
CH2Br2 Molecular Geometry - Science Education and Tutorials The formula of CH2Br2 molecular hybridization is as follows: No. Hyb of CH2Br2= N.A (C-H and C-Br bonds) + L.P (C) No. Hy of CH2Br2= the number of hybridizations of CH2Br2 Number of C-H and C-Br bonds = N.A (C-Br and C-H bonds) Lone pair on the central carbon atom = L.P (C) Calculation for hybridization number for CH2Br2 molecule
Identify the orbitals that overlap to form the C-Br bonds in CH2Br2 a ... Answer and Explanation: 1 In the molecule CH2Br2 C H 2 B r 2, the C atom is sp2 s p 2 hybridized. The Br atom has 4p orbital, with 1 electron, available for bonding. Thus, the sp2 s p 2 hybrid...
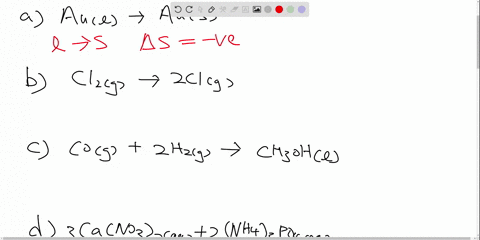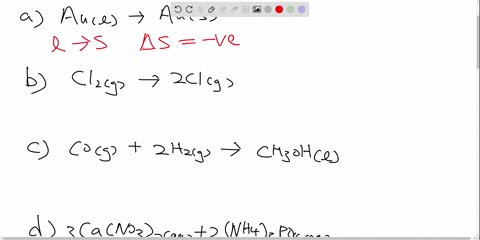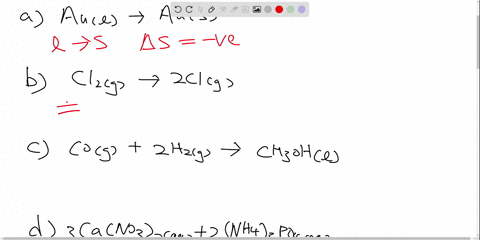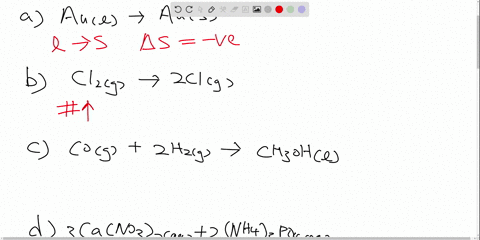
























Post a Comment for "42 ch2br2 hybridization"