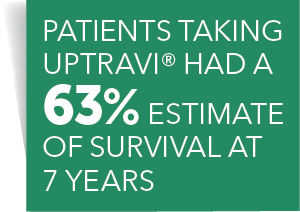
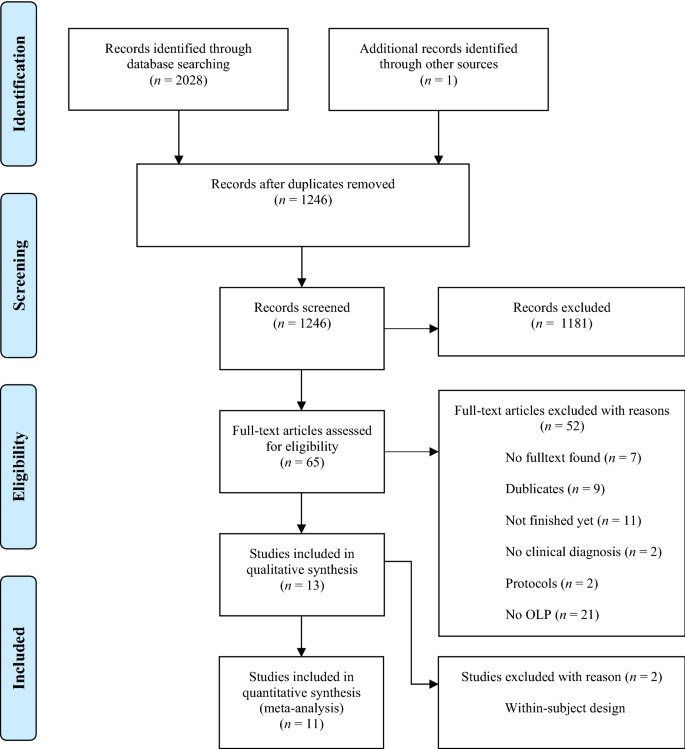
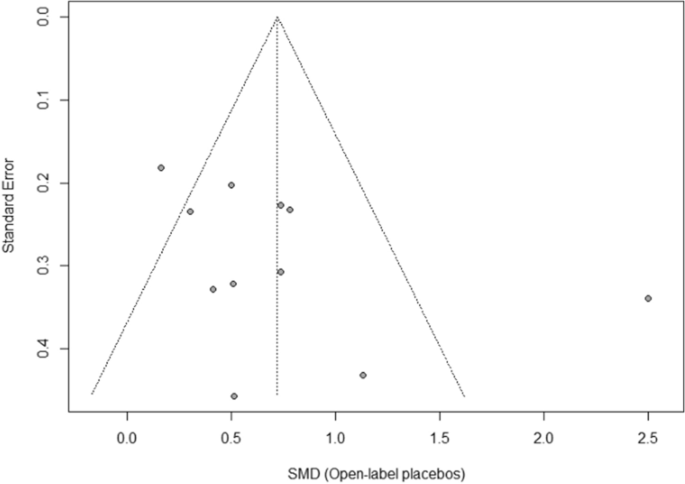
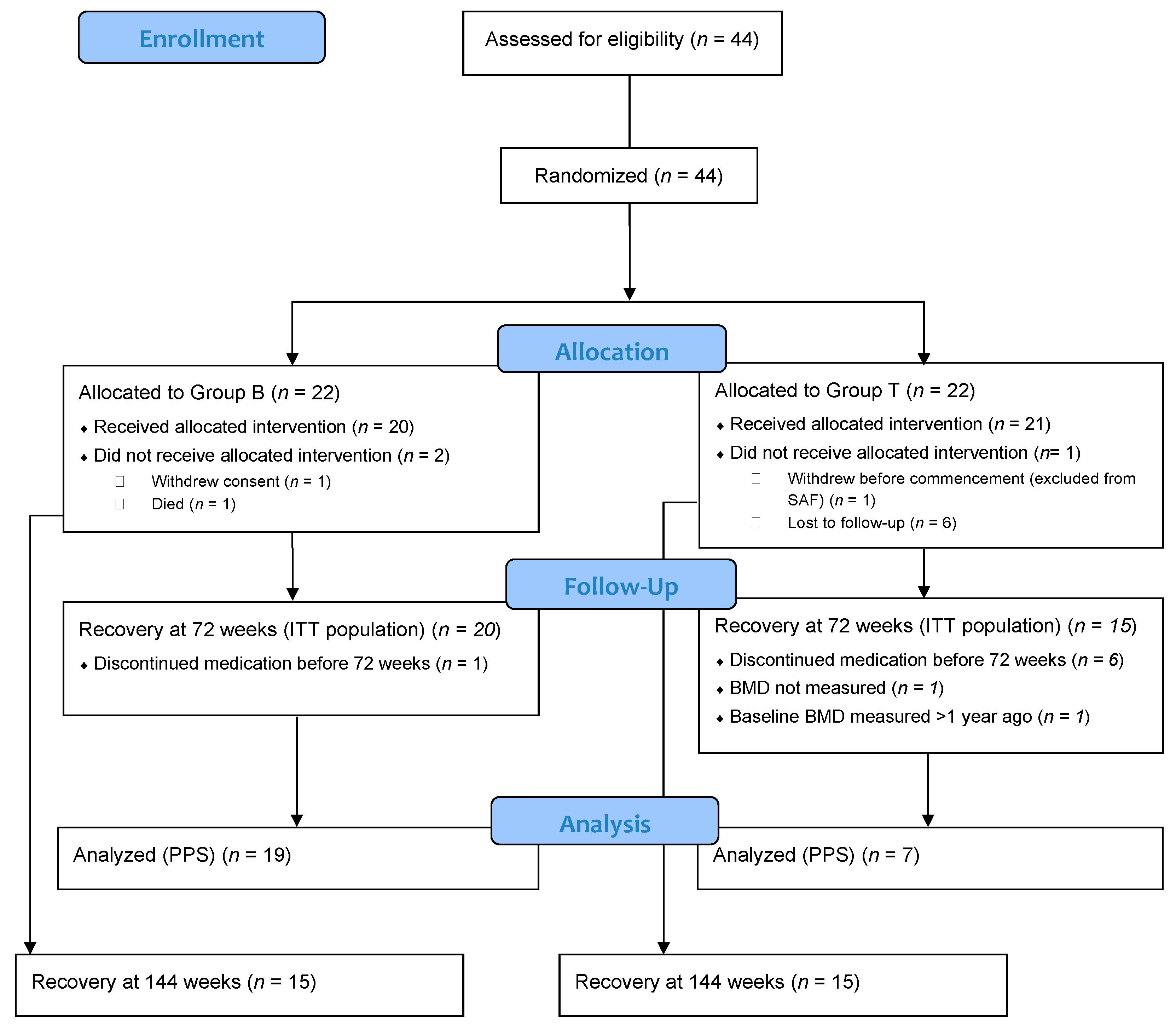
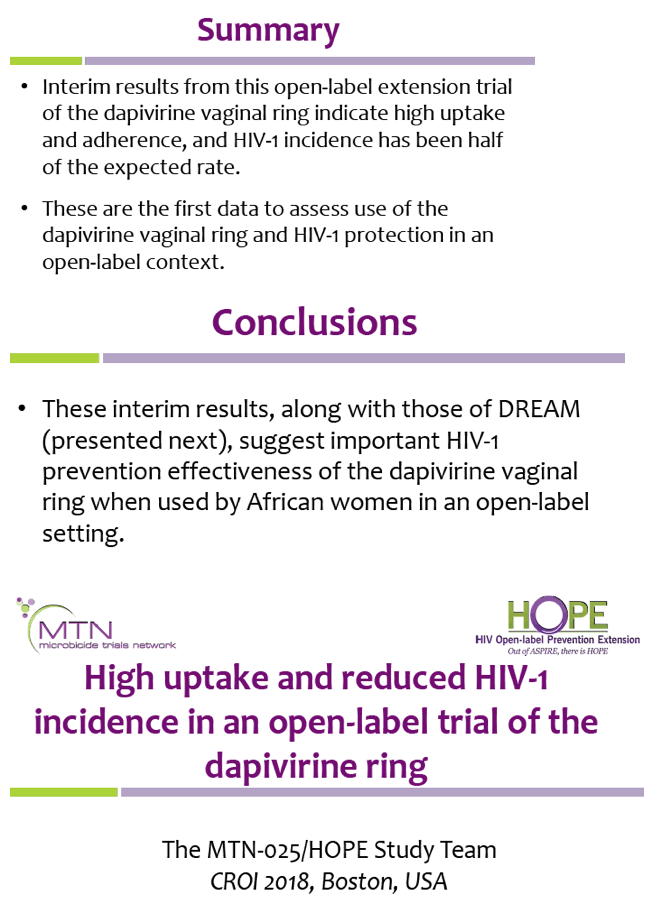
38 open-label study
en.wikipedia.org › wiki › Open-label_trialOpen-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. In particular, both the researchers and participants know which treatment is being administered. [1] clinicaltrials.gov › ct2 › showA Study to Evaluate the Effectiveness and Safety of IONIS-FB ... Jul 10, 2019 · This is a Phase 2, single arm open-label clinical study in up to 25 participants that will consist of a screening period, a 24-week treatment period, an optional treatment extension period of up to an additional 48 weeks, and a 12- week post-treatment follow-up evaluation period.
› journals › lanoncThe Lancet Oncology, November 2022, Volume 23, Issue 11 ... Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. François-Clément Bidard, Anne-Claire Hardy-Bessard, Florence Dalenc, Thomas Bachelot, Jean-Yves Pierga, Thibault de la ...

Open-label study
› health-topics › study-qualityStudy Quality Assessment Tools | NHLBI, NIH Was the study described as randomized, a randomized trial, a randomized clinical trial, or an RCT? 2. Was the method of randomization adequate (i.e., use of randomly generated assignment)? 3. Was the treatment allocation concealed (so that assignments could not be predicted)? 4. Were study participants and providers blinded to treatment group ... › journals › lancetCardiovascular outcomes in adults with hypertension with ... Oct 11, 2022 · Added value of this studyThe Treatment in Morning versus Evening (TIME) study was a large, pragmatic, decentralised, prospective, randomised, open-label, blinded-endpoint, superiority trial conducted in the UK, comparing cardiovascular outcomes in adults with hypertension randomly assigned to evening versus morning dosing of their usual ... clinicaltrials.gov › ct2 › showAn International Prospective Open-label, Randomized, Phase ... Jan 22, 2021 · An Open-label, Randomized, Phase III Study Comparing 177Lu-PSMA-617 in Combination With Standard of Care, Versus Standard of Care Alone, in Adult Male Patients With Metastatic Hormone Sensitive Prostate Cancer (mHSPC) Actual Study Start Date : June 9, 2021: Estimated Primary Completion Date : August 28, 2024: Estimated Study Completion Date :
Open-label study. › research › dgcgNIA Glossary of Clinical Research Terms | National Institute ... Adverse Event (AE) – Any untoward or unfavorable medical occurrence in a clinical research study participant, including any abnormal sign (e.g. abnormal physical exam or laboratory finding), symptom, or disease, temporally associated with the participants’ involvement in the research, whether or not considered related to participation in the research. clinicaltrials.gov › ct2 › showAn International Prospective Open-label, Randomized, Phase ... Jan 22, 2021 · An Open-label, Randomized, Phase III Study Comparing 177Lu-PSMA-617 in Combination With Standard of Care, Versus Standard of Care Alone, in Adult Male Patients With Metastatic Hormone Sensitive Prostate Cancer (mHSPC) Actual Study Start Date : June 9, 2021: Estimated Primary Completion Date : August 28, 2024: Estimated Study Completion Date : › journals › lancetCardiovascular outcomes in adults with hypertension with ... Oct 11, 2022 · Added value of this studyThe Treatment in Morning versus Evening (TIME) study was a large, pragmatic, decentralised, prospective, randomised, open-label, blinded-endpoint, superiority trial conducted in the UK, comparing cardiovascular outcomes in adults with hypertension randomly assigned to evening versus morning dosing of their usual ... › health-topics › study-qualityStudy Quality Assessment Tools | NHLBI, NIH Was the study described as randomized, a randomized trial, a randomized clinical trial, or an RCT? 2. Was the method of randomization adequate (i.e., use of randomly generated assignment)? 3. Was the treatment allocation concealed (so that assignments could not be predicted)? 4. Were study participants and providers blinded to treatment group ...


























Post a Comment for "38 open-label study"